Abstract
Introduction: The adverse events associated with hematopoietic stem cell donation have been extensively studied. There is an increasing literature linking psychological factors including stress, anxiety and depression to higher levels of inflammatory burden leading to poorer post-procedural outcomes including longer hospital stays and increased pain perception. Here, we aimed to evaluate whether pre-donation health related quality of life (HRQoL) markers predict toxicity profile and stem cell yield following stem cell donation in healthy donors.
Methods: The study population included adult granulocyte colony-stimulating factor (G-CSF) mobilized peripheral blood stem cell (PBSC) related donors (RD) (n= 157) and unrelated donors (URD) (n=179) who were enrolled in Related Donor Safety Study (RDSafe) and Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0201 clinical trials. Pre-donation HR QoL was assessed using the Short-Form (SF-8) in RDSafe and SF-12 questionnaire in BMT CTN 0201 (higher score is better). Pain and toxicity were collected on study specific forms. The primary outcome was the incidence of skeletal pain on day 5 of G-CSF administration. The secondary outcomes were the incidence of skeletal pain and highest toxicity level across selected body symptoms at 1 month, 6 months and 1-year post-donation. Another secondary outcome included CD34+ per liter of blood processed (x10 6/L) on day 5 of G-CSF as a measure of collection yield. The association between pre-apheresis HRQOL measures and pain and acute toxicities was characterized using means and SDs and compared using the t-test. Association between HRQoL and cell yield was assessed using the Pearson correlation coefficient. RD and URD were analyzed separately.
Results: URDs were younger than RDs (median age 35 vs. 63). A higher proportion of RDs were female (50% vs. 40%) and obese (41% vs. 35%). A higher proportion of RD PBSC donations required 2 days or more (44% vs 21%). More RDs were collected with lower volume procedures (<18L, 28% vs. 16%), and required a central line (28% vs. 11%). RDs were more likely to report pre-donation grade 1-2 pain (27% vs. 8%) and other toxicities (16% vs. 6%).
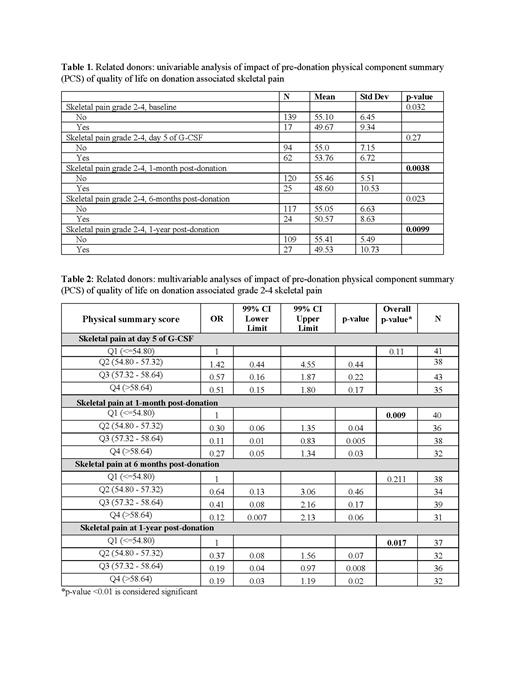
The mean pre-donation physical component summary (PCS) and mental component summary (MCS) score of RDs were 54.5 (SD 7.0) and 55.1 (SD 5.8), respectively . In the univariate analysis (table 1), pre-donation lower PCS score of RDs was associated with significantly more grade 2-4 pain at 1 month (p=0.0038) and 1-year post-donation (p=0.0099) (Table 1). In multivariable analysis (table 2), pre-donation PCS remained significantly associated with grade 2-4 pain 1-month post-donation (p=0.0098). More specifically, RDs with pre-donation PCS scores in the higher quartile were less likely to experience pain compared with donors with PCS scores in the lower quartile (OR 0.1; 95% CI 0.01-0.83; p=0.005). There was also a trend toward increased grade 2-4 pain at 1-year post-donation among RDs with lower PCS score (p=0.0176). Other outcomes such as pain at day 5 of G-CSF, other toxicities at day 5 of G-CSF, 1 month, 6 months and 1-year post-donation were not associated with pre-donation PCS score. Similarly, there was no significant association between RD pre-donation MCS score and collection-related symptoms at any time point.
The mean pre-donation PCS and MCS scores of URDs were 56.2 (SD 4.7) and 54.5 (SD 5.5), respectively . In a univariate analysis, there was no association between PCS score or MCS score and donation associated pain and toxicities at any time point post-donation. Due to low event rates, multivariable analysis was not performed in the URD setting. Based on the multivariable regression analysis, there was no correlation between pre-apheresis HRQoL score (PCS or MSC) and PBSC collection yield in either the RD or URD setting.
Conclusion: Our study demonstrates that pre-donation QoL markers are significantly associated with the toxicity profile after PBSC donation in the RD setting as adult RD with lower pre-donation physical QOL experience increased levels of pain after a PBSC collection procedure. There were no such associations found in URD in this small sample. Our findings may help clinicians to identify donors at higher risk of pain with donation, and lead to personalized information and interventions (e.g. increased analgesia) for specific donors. Future study with a larger sample is required to validate the results.
Farhadfar: Incyte: Consultancy. Stefanski: Novartis: Honoraria. Pulsipher: Equillium: Membership on an entity's Board of Directors or advisory committees; Adaptive: Research Funding; Jasper Therapeutics: Honoraria. Shaw: mallinkrodt: Other: payments; Orca bio: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal